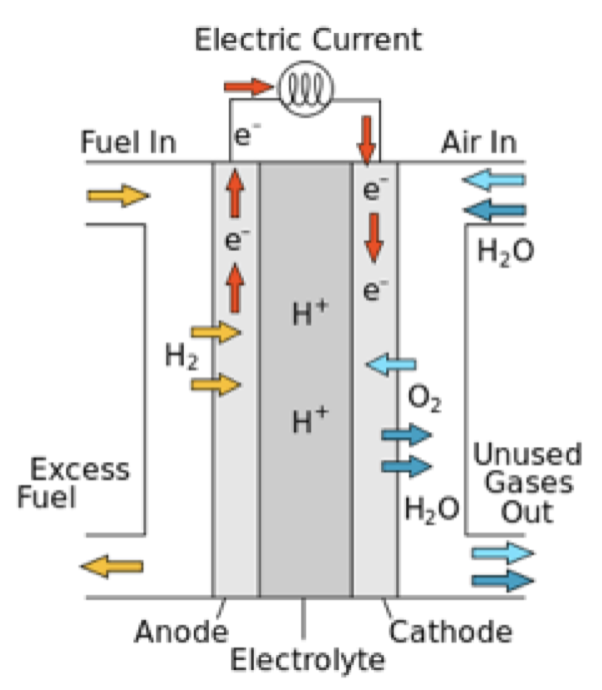
Chemicals Used In Fuel Cell . what is a fuel cell? This classification determines the kind of. fuel cells are classified primarily by the kind of electrolyte they employ. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. A fuel electrode (anode), an oxidant electrode (cathode), and an. — a fuel cell is composed of three active components: They produce electricity and heat as long as fuel is. Learn types of fuel cell, working and more here. fuel cells work like batteries, but they do not run down or need recharging. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available.
from studymind.co.uk
a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. Learn types of fuel cell, working and more here. A fuel electrode (anode), an oxidant electrode (cathode), and an. fuel cells work like batteries, but they do not run down or need recharging. — a fuel cell is composed of three active components: a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. what is a fuel cell? fuel cells are classified primarily by the kind of electrolyte they employ. This classification determines the kind of.
Fuel Cells (GCSE Chemistry) Study Mind
Chemicals Used In Fuel Cell fuel cells are classified primarily by the kind of electrolyte they employ. Learn types of fuel cell, working and more here. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. fuel cells work like batteries, but they do not run down or need recharging. A fuel electrode (anode), an oxidant electrode (cathode), and an. — a fuel cell is composed of three active components: a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. This classification determines the kind of. what is a fuel cell? It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. fuel cells are classified primarily by the kind of electrolyte they employ. They produce electricity and heat as long as fuel is.
From www.slideserve.com
PPT New Compound Materials for Fuel Cells PowerPoint Presentation Chemicals Used In Fuel Cell a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel electrode (anode), an oxidant electrode (cathode), and an. Learn types of fuel cell, working and more here. fuel cells work like batteries, but they do not run down or need recharging. . Chemicals Used In Fuel Cell.
From hydrogenpotanezu.blogspot.com
Hydrogen Oxygen Hydrogen Fuel Cell Chemicals Used In Fuel Cell a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. This classification determines the kind of. Learn types of fuel cell, working and more here. They produce electricity and heat as long as fuel is. — a fuel cell is composed of three active components: a fuel cell like. Chemicals Used In Fuel Cell.
From climatebiz.com
How To Build A DIY Hydrogen Fuel Cell Chemicals Used In Fuel Cell a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. A fuel electrode (anode), an oxidant electrode (cathode), and an. fuel cells work like batteries, but they do not run down or need recharging. — a fuel cell is composed of three active components: fuel cells are classified. Chemicals Used In Fuel Cell.
From www.alamy.com
Fuel cell and Liion battery diagram. Vector. Device that converts Chemicals Used In Fuel Cell Learn types of fuel cell, working and more here. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. — a fuel cell is composed of three active components: a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are. Chemicals Used In Fuel Cell.
From www.researchgate.net
Application of ALD on fuel cells. a) Different types of fuel cells with Chemicals Used In Fuel Cell fuel cells are classified primarily by the kind of electrolyte they employ. They produce electricity and heat as long as fuel is. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. a fuel cell like this will continue to operate and produce electrical energy as long as a. Chemicals Used In Fuel Cell.
From www.vrogue.co
Hydrogen Fuel Cell Design Life Cycle vrogue.co Chemicals Used In Fuel Cell a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. This classification determines the kind of. They produce electricity and heat as long as fuel is. Learn types of fuel cell, working and more here. — a fuel cell is composed of three active. Chemicals Used In Fuel Cell.
From www.britannica.com
Fuel cell Proton Exchange, Alkaline, Polymer Britannica Chemicals Used In Fuel Cell a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel electrode (anode), an oxidant electrode (cathode), and an. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. what is a fuel cell? This classification determines. Chemicals Used In Fuel Cell.
From studiousguy.com
Fuel Cell Working Principle StudiousGuy Chemicals Used In Fuel Cell a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. This classification determines the kind of. — a fuel cell is composed of three active components: A fuel. Chemicals Used In Fuel Cell.
From chem.libretexts.org
6.7 Batteries and Fuel Cells Chemistry LibreTexts Chemicals Used In Fuel Cell It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. what is a fuel cell? a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. A fuel electrode (anode), an oxidant electrode (cathode), and an. a fuel cell like this will continue to. Chemicals Used In Fuel Cell.
From pressbooks.bccampus.ca
5.6 Batteries and Fuel Cells Chemistry for Chemical Engineers Chemicals Used In Fuel Cell Learn types of fuel cell, working and more here. They produce electricity and heat as long as fuel is. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel electrode (anode), an oxidant electrode (cathode), and an. fuel cells are classified primarily. Chemicals Used In Fuel Cell.
From dxohmaewv.blob.core.windows.net
Bbc Chemical Cells And Fuel Cells at Lorraine Moore blog Chemicals Used In Fuel Cell fuel cells work like batteries, but they do not run down or need recharging. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. fuel cells are classified primarily by the kind of electrolyte they employ. This classification determines the kind of. a fuel cell like this will. Chemicals Used In Fuel Cell.
From www.researchgate.net
Schematic representation of Direct Methanol Fuel Cell (DMFC). This fuel Chemicals Used In Fuel Cell Learn types of fuel cell, working and more here. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. fuel cells are classified primarily by the kind of electrolyte they employ. They produce electricity. Chemicals Used In Fuel Cell.
From www.myelectrical2015.com
Electrical Revolution Alkaline Fuel Cell Advantages Limitations Chemicals Used In Fuel Cell Learn types of fuel cell, working and more here. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. — a fuel cell is composed of three active components: A fuel electrode (anode), an oxidant electrode (cathode), and an. fuel cells work like. Chemicals Used In Fuel Cell.
From iupac.org
Fuel Cells IUPAC International Union of Pure and Applied Chemistry Chemicals Used In Fuel Cell — a fuel cell is composed of three active components: fuel cells work like batteries, but they do not run down or need recharging. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. fuel cells are classified primarily by the kind of electrolyte they employ. a. Chemicals Used In Fuel Cell.
From www.tes.com
Electrolysis and Fuel Cells GCSE Chemistry Teaching Resources Chemicals Used In Fuel Cell — a fuel cell is composed of three active components: what is a fuel cell? They produce electricity and heat as long as fuel is. Learn types of fuel cell, working and more here. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are. Chemicals Used In Fuel Cell.
From cmsw.mit.edu
» Small Steps and Giant Leaps in Fuel Cell Technologies Angles / 2022 Chemicals Used In Fuel Cell what is a fuel cell? A fuel electrode (anode), an oxidant electrode (cathode), and an. fuel cells work like batteries, but they do not run down or need recharging. They produce electricity and heat as long as fuel is. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity.. Chemicals Used In Fuel Cell.
From chemistry.stackexchange.com
electrochemistry Electrode polarity in fuel cells Chemistry Stack Chemicals Used In Fuel Cell fuel cells work like batteries, but they do not run down or need recharging. — a fuel cell is composed of three active components: what is a fuel cell? This classification determines the kind of. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and. Chemicals Used In Fuel Cell.
From www.sundyne.com
Hydrogen Fuel Cells How Do They Work? Sundyne Chemicals Used In Fuel Cell a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. fuel cells work like batteries, but they do not run down or need recharging. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. They. Chemicals Used In Fuel Cell.